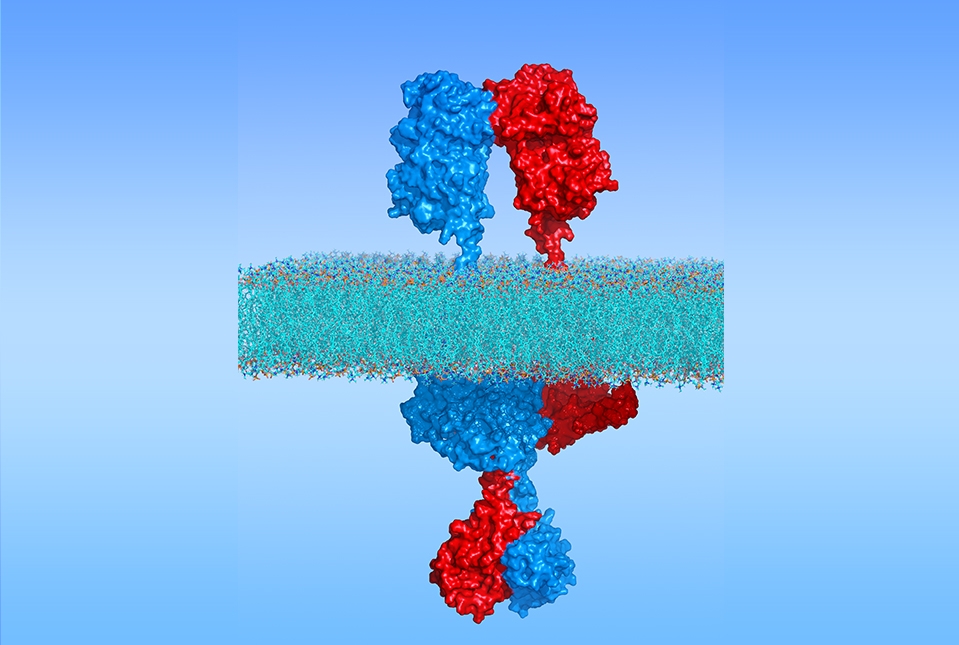
The full-length structure of the atrial natriuretic peptide receptor, pictured here, sheds light on how it functions in heart disease. Two copies of the receptor crossing the cell membrane (middle layer) are shown in red and blue. Credit: Huang Lab
Weill Cornell Medicine researchers have determined the full-length structure of a blood pressure-regulating hormone receptor for the first time, uncovering how it functions, which may enable better drug targeting of the receptor for diseases like hypertension and heart failure.
For the study, published Nov. 14 in Nature Structural & Molecular Biology, the researchers used cryo-electron microscopy (cryo-EM), computer modeling and other advanced techniques to determine the high-resolution structure of the atrial natriuretic peptide receptor 1, also known as transmembrane receptor guanylyl cyclase A (GC-A).
GC-A is a type of single-pass transmembrane receptor protein that spans the cell membrane, connecting a binding region on the cell surface to an interior signaling domain, allowing it to transmit signals from outside the cell to the inside. Basically, the receptor acts as a communication channel between the cell and its environment.
“These new structural details will be of interest to drug developers who want to target GC-A and related receptors to treat heart disease and other conditions,” said study senior author Dr. Xin-Yun Huang, professor of physiology and biophysics at Weill Cornell Medicine. Read more…